还记得那道考过不知道多少遍的化学题吗?
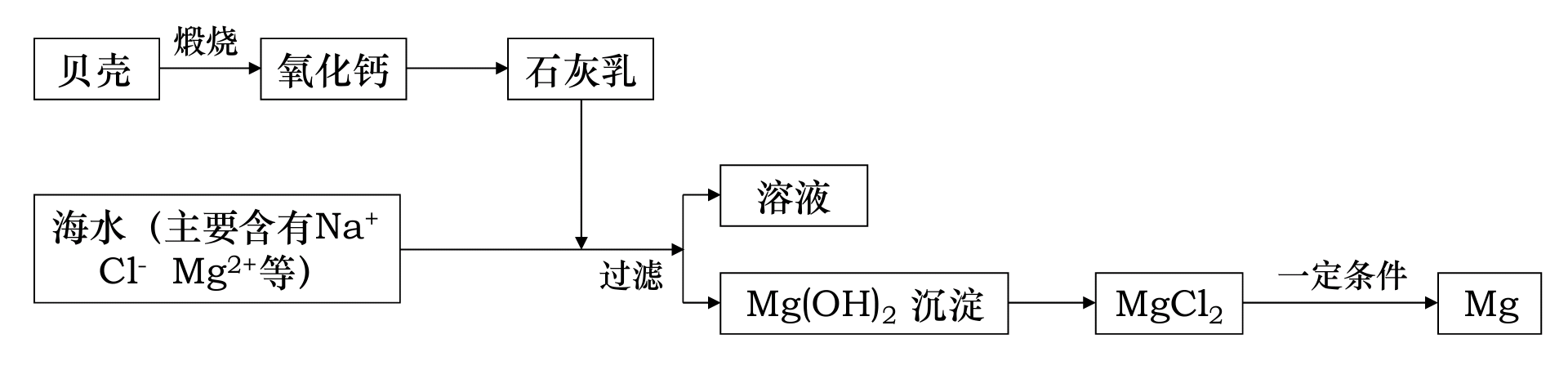
做了那么多遍,工业怎么制镁,你记住了吗?
镁,被称为“国防金属”,工业上,常采用电解水溶液的方法来制取镁。一个制镁厂,通常由以下几部分构成:
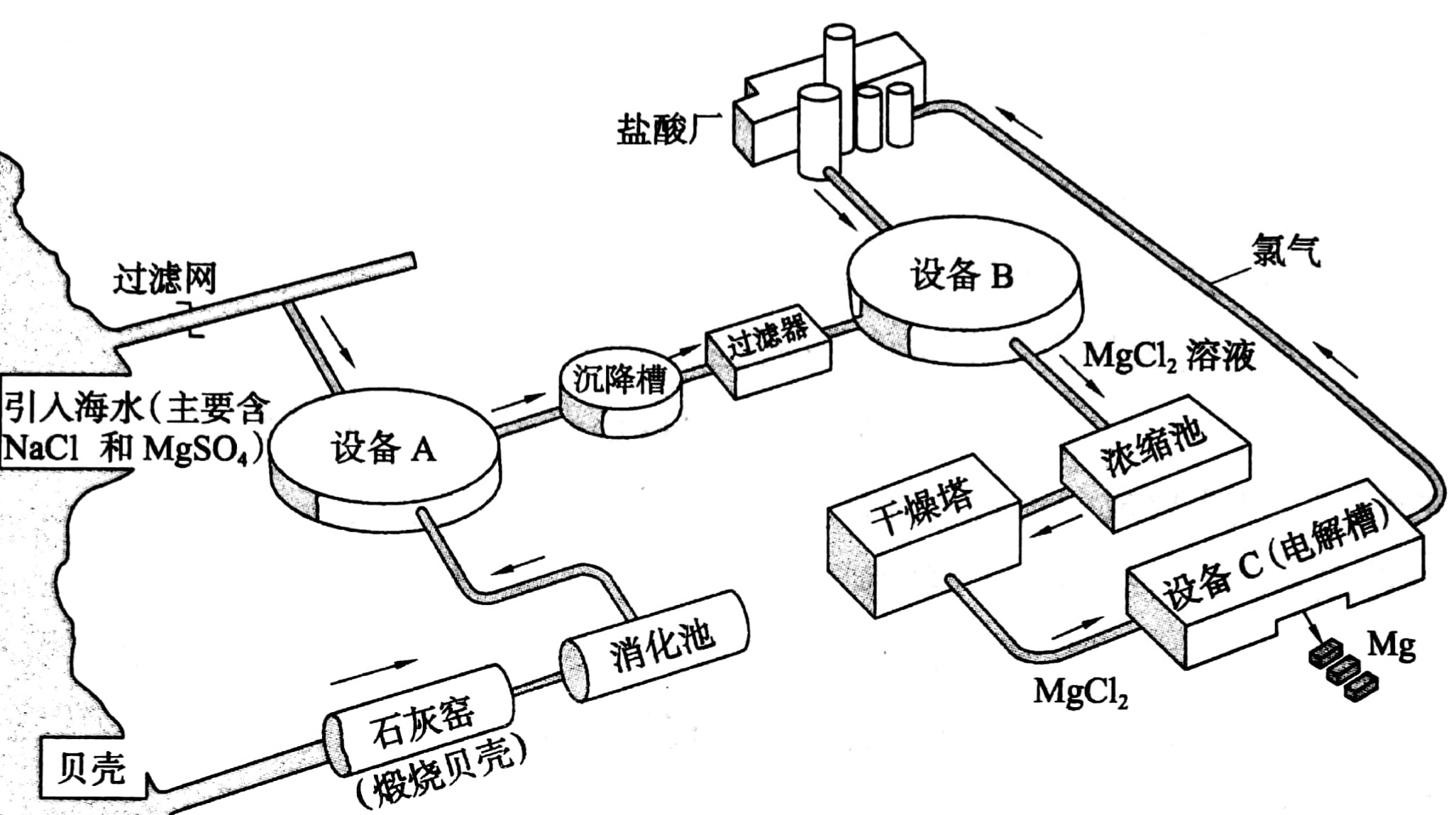
首先,在石灰窑将海边大量存在的贝壳煅烧成生石灰,方程式为。
然后,在消化池中把生石灰转化成熟石灰,方程式为。
在设备A中把熟石灰与过滤后的海水混合,生成不溶于水的沉淀,方程式为。
把过滤后的沉淀置于设备B中,加入,通过反应把转化为,浓缩、干燥后生成结晶。
加热结晶,得到无水,进行电解,即可得到金属镁,反应的方程式为,产生的氯气回收利用,通入盐酸厂,与水反应产生盐酸,回到设备B参与反应,实现了有毒物的回收,节约了资源。
通过该过程,实现了海水中镁元素的富集和提纯;但生产过程中需耗费大量电能,不环保,人们又想到很多办法制镁,如下表所示:
| 技术 | 原料 | |
| 电解法 | 道乌法 | 海水卤水 |
| 氧化镁氯化法 | 菱镁矿 | |
| 光卤石法 | 光卤石 | |
| AMC法 | 盐湖卤水 | |
| 诺斯克法 | 海水卤水 | |
| 硅热法 | 皮江法 | 白云石 |
| 玻尔扎诺法 | ||
| 半连续法 | ||
Do you remember the chemistry problem that you have done it for many time?

You’ve done that many times. How does the industry(工业) produce magnesium(镁)? Do you remember?
Magnesium, known as “defense metal”, in industry, we produce magnesium by electrolysis of ’s water solution(溶液). A magnesium factory usually has the following parts:

First of all, convert the common shells into quick lime(生石灰) in the lime kiln, the equation is .
Then, the lime is converted into mature lime in the digester, and the equation is .
In equipment A, the mature lime is mixed with filtered(过滤) seawater to produce , which is insoluble(不溶的) in water, and the equation is .
The filtered will be put in equipment B, then add , through reaction the has been converted into , afterconcentration and drying, we generated .
Heat the crystals, get anhydrous(无水的) , with electrolysis, we can get metal magnesium, the equation is , for chlorine(氯气) recycling, it will be sent into hydrochloric acid(盐酸) factory, react with water and produce hydrochloric acid, then back to equipment B to participate in the reaction, realize the recycling of toxins(有毒物质), and save the resources.
Through this process, the magnesium in seawater is enriched. However, in the process, a large amount of electricity is needed, which is not good for environment. People also think of a lot of ways to process magnesium, as shown in the following table:
| Technique | Material | |
| 电解法 | 道乌法 | 海水卤水 |
| 氧化镁氯化法 | 菱镁矿 | |
| 光卤石法 | 光卤石 | |
| AMC法 | 盐湖卤水 | |
| 诺斯克法 | 海水卤水 | |
| 硅热法 | 皮江法 | 白云石 |
| 玻尔扎诺法 | ||
| 半连续法 | ||